
“Early diagnosis of proteinopathies using massively parallel nano-spectroscopy with single-molecule sensitivity. Advanced clinical diagnostics for the development of personalized treatments”
Path to Precision Medicine project number: PPM 04-0131-200019
April 15th 2021 – April 15th 2024.
About the project
The project “Early diagnosis of proteinopathies using massively parallel nano-spectroscopy with single-molecule sensitivity. Advanced clinical diagnostics for the development of personalized treatments” is funded by the Qatar National Research Fund under grant number PPM 04-0131-200019.
The all-encompassing goal of this research project is to develop specialized photonics instrumentation based on the massively parallel Fluorescence Correlation Spectroscopy (mpFCS), mathematical analysis tools, and dedicated software that will allow us to translate our innovative method for ultrasensitive detection of structured peptide/protein aggregates to the primary care and use it to diagnose devastating proteopathy diseases with the ultimate, single-molecule sensitivity.
Aims of the project
- Design and development of a compact, table-top instrument for mpFCS that will allow medical professionals to identify by screening individuals with elevated levels of structured peptide/protein aggregates in the blood serum, who are at risk to develop/have developed a proteopathy disease,
- Design and development of a compact, table-top instrument for dual color mpFCS that will allow medical professionals to specifically diagnose the proteopathy disease in individuals who are identified by screening to have elevated levels of structured protein aggregates,
- New innovative software for full control of single and dual color table-top instruments with fast acquisition, analysis and representation of large data sets.
Research methods
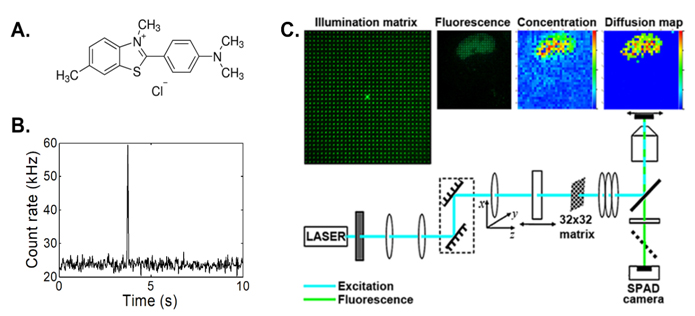
The idea to translate Fluorescence Correlation Spectroscopy (FCS), a quantitative analytical method with the ultimate, single-molecule sensitivity at room temperature [1] to healthcare, for rapid and cost-effective identification of individuals with elevated levels of structured peptide/protein aggregates in the blood serum (or other biological fluids), stems from our recent innovative work [2-4]. As a first step, we have shown that FCS in combination with Thioflavin T (ThT, figure A) is uniquely applicable for the detection of ThT-responsive structured aggregates in vitro [2] and in biological fluids (figure B) [3]. As a second step, we have successfully extended FCS to imaging and developed instrumentation for functional Fluorescence Microscopy Imaging (fFMI) by deploying simultaneous multi-point FCS [4] (figure C). Here, a Diffractive Optical Element (DOE) is used to generate an illumination matrix of 64×32, 32×32, or 16×16 spots that perfectly matches a 2D single-photon avalanche diode (SPAD) array, to simultaneously collect the signal in 1024 observation volume elements. This allows us to map the concentration, diffusion and velocity map of molecules of interest.
References
- Krmpot AJ, Nikolić SN, Oasa S, Papadopoulos DK, Vitali M, Oura M, Mikuni S, Thyberg P, Tisa S, Kinjo M, Nilsson L, Terenius L, Rigler R, Vukojević V.:
“Functional Fluorescence Microscopy Imaging (fFMI). Quantitative Scanning-Free Confocal Fluorescence Microscopy for the Characterization of Fast Dynamic Processes in Live Cells”,
Analytical Chemistry 91, 11129-11137 (2019). - Tiiman A, Jelić V, Jarvet J, Järemo P, Bogdanović N, Rigler R, Terenius L, Gräslund A, Vukojević V.:
“Amyloidogenic nanoplaques in blood serum of patients with Alzheimer’s disease revealed by time-resolved Thioflavin T fluorescence intensity fluctuation analysis”,
J. Alzheimer’s Dis. 68, 571-582 (2019). - Tiiman A, Jarvet J, Gräslund A, Vukojević V.:
“Heterogeneity and intermediates turnover during amyloid-β (Aβ) peptide aggregation studied by Fluorescence Correlation Spectroscopy”,
Biochemistry 54, 7203-7211 (2015). - Bonito-Oliva A, Schedin-Weiss S, Younesi SS, Tiiman A, Adura C, Paknejad N, Brendel M, Romin Y, Parchem RJ, Graff C, Vukojević V, Tjernberg LO, Terenius L, Winblad B, Sakmar TP, Graham WV.:
“Conformation-specific antibodies against multiple amyloid protofibril species from a single amyloid immunogen”,
J Cell Mol Med. 23, 2103-2114 (2019).
Published papers with acknowledgement to the PPM project
- Sho Oasa, Aleksandar J. Krmpot, Stanko N. Nikolić, Andrew H. A. Clayton, Igor F. Tsigelny, Jean-Pierre Changeux, Lars Terenius, Rudolf Rigler, and Vladana Vukojević:
“Functional Fluorescence Microscopy Imaging (fFMI). Quantitative Scanning-Free Confocal Fluorescence Microscopy for the Characterization of Fast Dynamic Processes in Live Cells”, Analytical Chemistry 91: 11129-11137 (2019). - Mihajlo D. Radmilović, Ivana T. Drvenica, Mihailo D. Rabasović, Vesna Lj. Ilić, Danica Pavlović, Sho Oasa, Vladana Vukojević, Mina Perić, Stanko N. Nikolić, Aleksandar J. Krmpot: “Interactions of ultrashort laser pulses with hemoglobin: Photophysical aspects and potential applications”, International Journal of Biological Macromolecules 244, 125312 (2023).
- Stanko N. Nikolić, Sho Oasa, Aleksandar J. Krmpot, Lars Terenius, Milivoj R. Belić, Rudolf Rigler, Vladana Vukojević:
“Mapping the direction of nucleocytoplasmic transport of glucocorticoid receptor (GR) in live cells using two-foci cross-correlation in massively parallel Fluorescence Correlation Spectroscopy (mpFCS)”, Analytical Chemistry 95, 15171-15179 (2023).
Project team

Dr. Milivoj R. Belić (Lead PI)
Office Phone: +974.4423.0124
Email: milivoj.belic@qatar.tamu.edu
Address: Texas A&M University at Qatar
Education City, Doha, Qatar

Dr. Vladana Vukojević (PI)
Office Phone: +46-(0)8-517 717 22
Email: vladana.vukojevic@ki.se
Department of Clinical Neuroscience
Karolinska Institutet
Stockholm, Sweden

Dr. Aleksandar J. Krmpot
Postdoctoral Research Associate
Email: aleksandar.krmpot@qatar.tamu.edu
Address: Texas A&M University at Qatar
Education City, Doha, Qatar

Dr. Stanko N. Nikolić
Postdoctoral Research Associate
Email: stanko.nikolic@qatar.tamu.edu
Address: Texas A&M University at Qatar
Education City, Doha, Qatar
Selected activities during the second year of the project (end of M24)
- Dr. Stanko Nikolić and Dr. Aleksandar Krmpot went for a scientific mission to KI in Stockholm (Sweden), from January 27th, 2023 until February 10th, 2023. The goal of this visit was to work and finalize the project tasks during the second year with PI Assoc. Prof. Vladana Vukojević and other colleagues from KI. During their stay, Dr. Nikolić and Dr. Krmpot worked on the further setting up and improvement of the single-color and dual-color mpFCS instruments and associated software. The LPI Prof. Belić and PI Prof. Vladana Vukojević have assessed the visit as highly successful since the final stages of the deliverables WP2-D1, WP2-D2, WP3-D6, and WP3-D7 were finished during this visit. In addition, Prof. Belić, Prof. Vukojević, Dr. Krmpot, and Dr. Nikolić held seminars and discussions with KI colleagues about current project progress and pave the way for execution of project’s tasks in the third year. Prof. Belić was attending these events online since he was unable to travel to Stockholm due to teaching duties at TAMUQ.
Selected activities during the first half of the third year of the project (end of m30)
- The use of the single-color massively parallel Fluorescent Correlation Spectroscopy (mpFCS) instrument in a clinical/primary care setting in the protocols for early diagnostics of different proteinopathy diseases was conducted. The suggestions for protocols for early diagnostics of Alzheimer’s disease were proposed. The Work Package 1 of the PPM project is successfully finished with all goals from the Research Plan accomplished.
- The testing of the dual-color mpFCS instrument with shortcomings was identified and the improved instrument prototype was suggested. The user-manual for operating the mpFCS experimental system with two lasers was also provided.
- The new software add-in tools for single color and dual-color mpFCS instruments were developed. The programming modules enable the raw photon counts visualization, determining the average autocorrelation function over selected and non-selected pixels in the dual-color mpFCS setup, calculation of cross-correlation functions after photobleaching correction, and statistics of peaks in raw photon counts matrix.
- Dr. Aleksandar Krmpot and Dr. Stanko Nikolic attended the IX International School and Conference on Photonics, August 28 – September 01, 2023, in Belgrade, Serbia. During many informal talks with experts in the biophotonics field, Dr. Krmpot and Dr. Nikolic discussed the problems and challenges in the further development of the table-top dual-color mpFCS instruments at Karolinska Institute (KI). The conclusion is that potential collaboration between TAMUQ and KI with other prestigious universities and institutes can be established on this ground.
Selected activities during the second half of the third year of the project (end of M36)
- The use of the dual-color massively parallel Fluorescent Correlation Spectroscopy (mpFCS) instrument in a clinical/primary care setting in the protocols for early diagnostics of different proteinopathy diseases was conducted. The suggestions for protocols for early diagnostics of Alzheimer’s disease were proposed. The Work Package 2 of the PPM project is successfully finished with all goals from the Research Plan accomplished.
- The new software add-in tools for single color and dual-color mpFCS instruments were developed. The programming modules enable the data analysis in terms of massively parallel fitting of all auto-and cross-correlation functions to four different diffusion models and comprehensive statistical analysis of large dataset in the mpFCS experiment. The user can now compute the molecular diffusion times, concentrations, and two-dimensional velocities maps over entire detection matrix. The Work Package 3 of the PPM project is successfully finished with all goals from the Research Plan accomplished.
- Associate Professor Vladana Vukojević (PI) and Assistant Professor Sho Oasa (participant of the PPM project), both from Karolinska Institutet, Department of Clinical Neuroscience in Stockholm, visited the Texas A&M University at Qatar from March 26th until March 31st, 2024. Professor Milivoj Belić, the LPI of the project, hosted the colleagues from Sweden and organized the final project meeting at TAMUQ on March 27th. Dr. Stanko Nikolić and Dr. Aleksandar Krmpot, Postdoctoral Research Associates at TAMUQ and PPM participants, also attended this event (Dr. Nikolić in-person and Dr. Krmpot online). Participants agreed that all project tasks were successfully implemented and agreed to continue the scientific collaboration in the future.

The final PPM project meeting at TAMUQ on March 27th, 2024. From left to right: Asst. Prof. Sho Oasa, Dr. Stanko Nikolić, Assoc. Prof. Vladana Vukojević, Prof. Milivoj Belić, and Dr. Aleksandar Krmpot (on the screen)
- Associate Professor Vladana Vukojević held a lecture “Fluorescence Correlation Spectroscopy – Theoretical Background and Applications in Biomedical Research” at TAMUQ on March 28th, 2024. In the first part of the talk, Dr. Vukojević provided a brief overview of the Fluorescence Correlation Spectroscopy (FCS) technique and its applications in various scientific fields. The second part of the talk was dedicated to the accomplished goals of the PPM project. Dr. Vukojević presented how ultrasensitive FCS detection of structured peptide/protein aggregates with single-molecule sensitivity can help in diagnosing devastating proteopathy diseases in primary care settings.

Talk by Assoc. Prof. Vladana Vukojević at TAMUQ on March 28th, 2024